Cookie Settings
We use cookies to provide an optimal experience for you. Technically required cookies are used for making shopping possible, statistics are used for anonymized Google Analytics. You can read everything in our updated privacy policy.
| Sample material | 10 ml human buffy coat |
| Isolation method | Non-magnetic with 280µl CD14 S-pluriBead® anti-hu or 300µl M-pluriBead® anti-hu |
| Yield | ~6 * 10^6 S-pluriBead® or ~12 * 10^6 M-pluriBead® |
| Vitality | >92% (Trypan blue staining) |
| Purity | ~97% |
Wash sample material twice with provided Washing Buffer (PBS + 0,05 % BSA and 2 mM EDTA, pH 7,4) in order to reduce soluble CD14. Add pluriBead CD14 for isolation of monocytes into the sample tube and incubate on a pluriPlix or wiping rolling mixer for 20 minutes.
After isolation, resuspend the cells in 1ml of RPMI 1640-medium (+10 % FCS and 1x Pen/Strep) and determine the cell number. For the cultivation of monocytes in a 24-well cell culture plate, 1 million cells per well are used and incubated with 1ml monocytes-culture-medium (RPMI 1640 + 10 % FCS, 1x Pen/Strep, 2000 U/ml GM-CSF und 200 U/ml IL-4) at 37 °C and 5 % carbon dioxid.
After 24h, remove the medium and add 1ml fresh monocytes-culture-medium. Then, the cells are cultured for 4 more days. After a total of 5 days, stimulate the cells with 100ng/ml LPS for another 24h. The activated cells are then removed with trypsin and the maturation rate to dentritic cells from monocytes is detected by fluorescent-analysis of CD1a, CD14 and CD83.
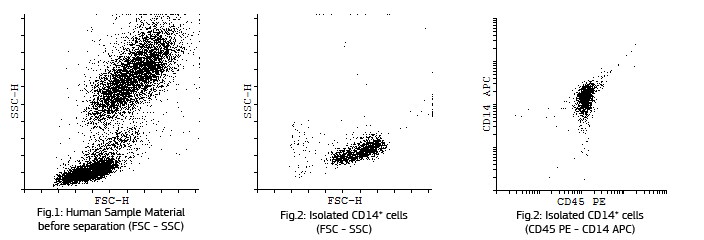
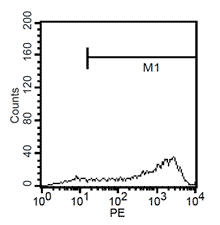
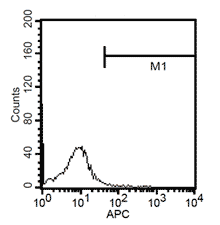
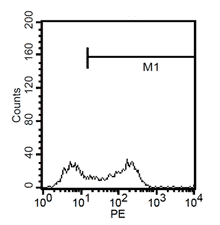
Fluorescent analysis of mature dentritic cells CD11a+, CD14+, CD83+
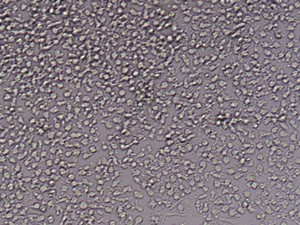
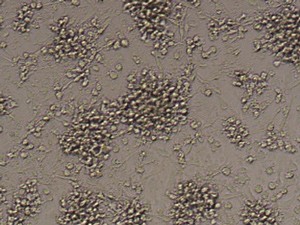
CD14+ cells after isolation in culture, Maturation of monocytes to dendritic cells after 5 days of culture with GM-CSIF/IL-4 and stimulation with LPS